Functional Service Provision
GEM typically collaborates with Statistical experts and experienced Medical Writers to deliver a full biometrics solution to report your study.
Since incorporation GEM has reported several phase I and II studies, across multiple therapeutic areas. In addition to standard study reporting, GEM has also undertaken: –
- CDISC legacy conversions
- Pooled safety and efficacy reporting
- Post hoc regulatory analyses
- DSMB/SRM unblinding
The GEM team has a very strong background in oncology, both early and late phase, but also has significant experience of other therapy areas, including immunology, infection, cardiovascular, CNS and respiratory.
Our Leadership Team have long been proponents of industry standards and we are a gold sponsor of the CDISC organisation. CDISC compliance underpins everything we deliver at GEM. With a growing in-house standards code library, we can deliver your study quickly and efficiently.
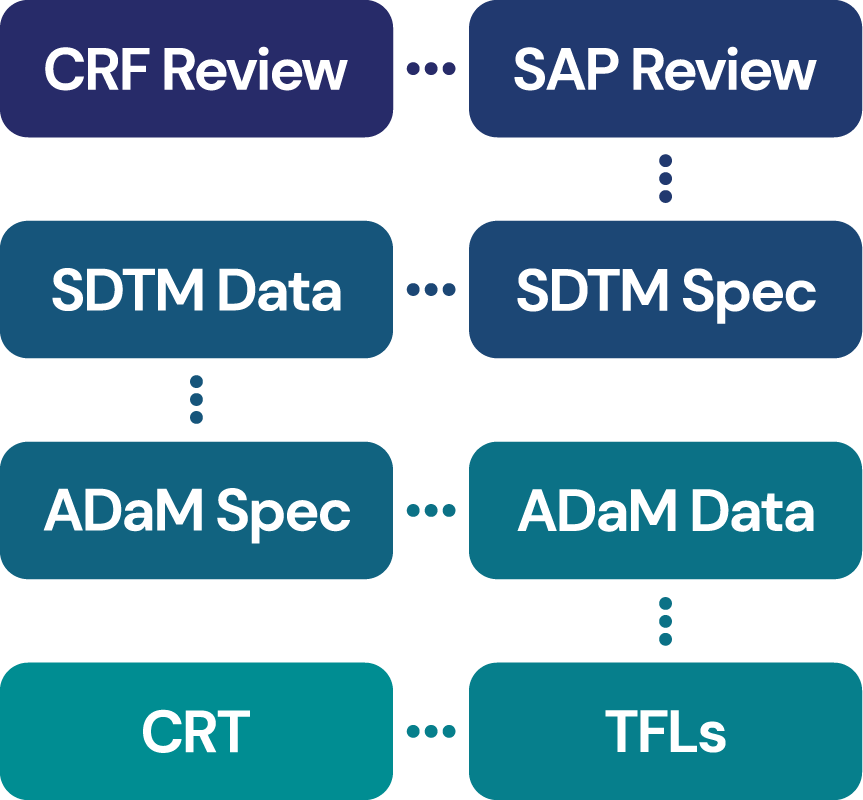
GEM can deliver all your study reporting requirements including: –
- CRF and database review including Data Management Plan
- CDISC SDTM specifications and dataset creation
- CDISC ADaM specifications and dataset creation
- Tables, Figures and Listings in text, RTF [Microsoft Word], PDF, Microsoft Excel, PowerPoint or any combination
- CRT package (Define.xml, SAS transport files, Reviewers Guide, aCRF)
- Patient profiles – e.g. to support dose escalation decisions
Our programmers are passionate about delivering high quality data and our comprehensive validation process ensures that all deliverables are risk assessed to select the most relevant validation methodologies. Any output identified as high-risk – typically all datasets and summary tables, will be independently coded by a second programmer as well as undergoing a detailed visual inspection.
We regularly work with trusted Clinical experts to deliver a full biometrics solution to report your study.
Contact us to learn more about our GEM study reporting solutions.
